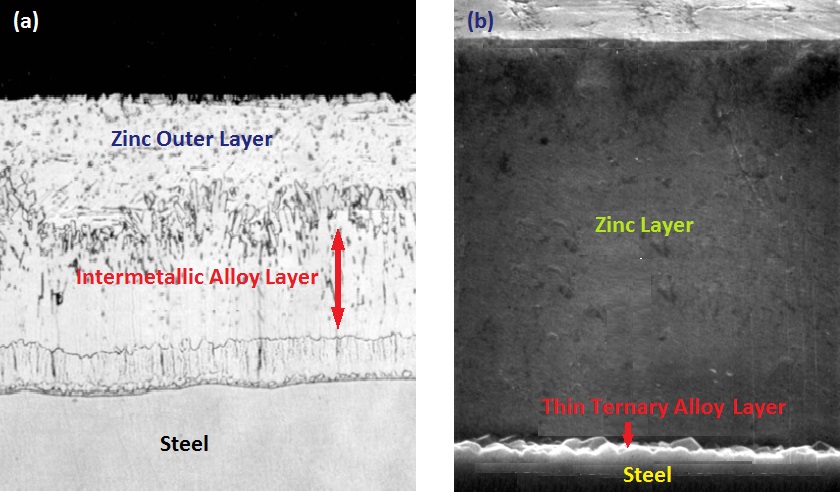
The aluminium free coating forms Zn- Fe intermetallic compounds between the steel surface and the pure zinc outer layer. The formation of these phases provides a strong adhesion between the coating and the base metal. However, the Zn-Fe intermetallic compounds are very brittle, which results in shear cracks developing during forming of steel sheets and is considered as one of the main causes for flaking of the protective coating, a common defect observed in galvanized products. The coating embrittlement drastically limits the forming ability of steel sheets.
To overcome the issue of embrittlement in galvanizing coatings, small concentrations of aluminium is added to the molten zinc bath. Aluminium addition to the galvanizing bath restricts the growth of the intermetallic layer by inhibiting the reaction between zinc and iron, thus forming a considerably thinner intermetallic layer compared to an aluminium free coating. The thickness reduction of the intermetallic phase enhances the ductility of the coating. As a consequence, the galvanized steel sheet products can be formed into more complex shapes without the loss of coating adhesion or the formation of internal shear cracks. The aluminium content in continuous galvanizing baths is normally kept to ~0.15-0.19 %, while some galvanizers use higher Al concentrations (as much as 0.20 to 0.25 %) in their continuous galvanizing lines. While aluminium addition improves the formability of steel sheets, it has only a negligible effect on the other properties of the products. For instance, the bulk corrosion resistance of the material is not affected and although, other properties such as soldering, spot welding, and white rusting is adversely affected by the presentence of Al, it is still tolerable considering the great benefit of Al addition in enhancing sheet formability of galvanized products.
Without the presence of aluminium, the intermetallic compound, FeZn7, is the most chemically stable phase that forms immediately after submersion of steel into the bath. Aluminium, however, has a much more affinity for iron compared to zinc and hence, an aluminium-iron intermetallic layer, Fe2Al5, is preferentially formed. This extremely thin layer inhibits the reaction between zinc and iron. During the fast galvanizing step of ~2-4 sec, a thin layer of ternary alloy, Fe2Al5-xZnx, is formed on top of this barrier layer. This layer is typically composed of 45% Al, 35% Fe, and 20-35 % Zn. The growth rate of this layer is dependent upon the diffusion characteristics of zinc through the barrier layer. As a result, the growth of this layer is much slower than the aluminium free galvanizing system. The thin layer of ternary alloy formed due to Al addition is shown in figure 1(b).
An interesting observation in the analysis of aluminium containing galvanizing is that the concentration of this element in the coating is 0.25-0.4 %, which is substantially higher than the concentration of the molten bath (0.15-0.17 %). This is due to the accumulation of aluminium at the steel surface. The high affinity of aluminium for iron causes a high amount of aluminium to be removed from the bath together with the steel sheet in the form of Fe2Al5 barrier layer. Since the thickness of the barrier layer is independent of the coating mass, a lighter coating contains higher Al concentrations. Therefore, a light sheet with a high surface area has more Al content compared to a heavy steel component with a thicker coating.
